|
|
(E)-1,1,1-trichloro-3-nitroprop-2-ene
|
|
|
|
|
Substance index - selected nitrocompounds tested by our group /
|
|
CAS: 763-16-6
MW: 190.413
Brutto formula: C3H2NO2Cl3
B.p. [oC]: 88-89/16mmHg [Ref.]
Density [g/cm3]: 1.5695 (20oC)[Ref.]
IR [cm-1]: 1659, 1542, 1358 [Ref.]
|
Preparation [Ref.]:
|
|
To 350 g of 1,1,1-tri-chloro-2-acetoxy-3-nitropropane dissolved in 2400 mL of benzene was added 150 g. of powdered sodium carbonate monohydrate. After refluxing vigorously for 0.5 hour and allowing to cool, the mixture was filtered and the solvent distilled. Distillation of the residue under reduced pressure afforded 244 g (90.5%) of yellow, oily, lachrymatory liquid.
|
Applications:
|
|
|
|
 |
|
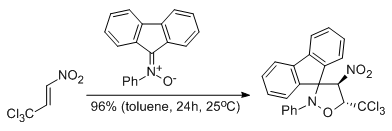
Experimental and theoretical DFT study on synthesis of sterically crowded 2,3,3,(4)5-tetrasubstituted-4-nitroisoxazolidines via 1,3-dipolar cycloaddition reactions between ketonitrones and conjugated nitroalkenes
Jasiński R., Mróz K., Kącka A.
Journal of Heterocyclic Chemistry, 53, 1424 (2016) |
|
|
|
|
 |
|
Kinetic aspects of [3+2] cycloaddition reactions between (E)-3,3,3-trichloro-1-nitroprop-1-ene and ketonitrones
Jasiński R., Mróz K.
Reaction Kinetics, Mechanisms and Catalysis, 116, 35-41 (2015) |
|
|
|
|
|
|
 |
|
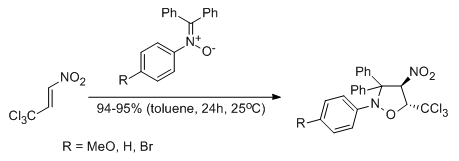
Experimental and theoretical DFT study on synthesis of sterically crowded 2,3,3,(4)5-tetrasubstituted-4-nitroisoxazolidines via 1,3-dipolar cycloaddition reactions between ketonitrones and conjugated nitroalkenes
Jasiński R., Mróz K., Kącka A.
Journal of Heterocyclic Chemistry, 53, 1424 (2016) |
|
|
|
|
 |
|
Kinetic aspects of [3+2] cycloaddition reactions between (E)-3,3,3-trichloro-1-nitroprop-1-ene and ketonitrones
Jasiński R., Mróz K.
Reaction Kinetics, Mechanisms and Catalysis, 116, 35-41 (2015) |
|
|
|
|
|
|
 |
|
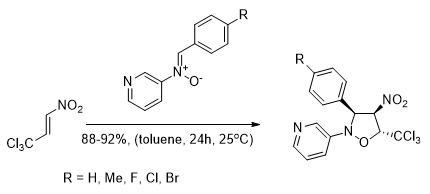
Regio- and stereoselective synthesis of nitro-functionalized analogs of nicotine
Fryzlewicz A., Łapczuk-Krygier A., Kula K., Demchuk O.M., Dresler E., Jasiński R.
Chemistry of Heterocyclic Compounds, 56, 120 (2020) |
|
|
|
|
|
|
 |
|
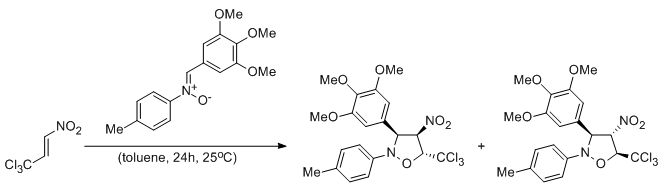
An experimental and quantumchemical study of [2+3] cycloaddition between (Z)-C-(m,m,p-trimethoxyphenyl)-N-(p-methyphenyl)-nitrone and (E)-3,3,3-trichloro-1-nitroprop-1-ene: mechanistic aspects
Szczepanek A., Jasińska E., Kącka A., Jasiński R.
Current Chemistry Letters, 4, 33-44 (2015) |
|
|
|
|
|
|
 |
|
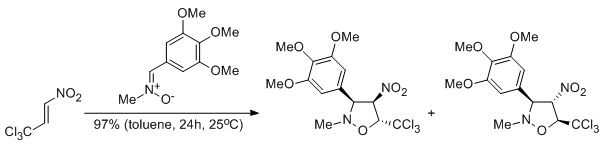
Regio- and stereoselectivity of polar [2+3] cycloaddition reactions between (Z)-C-(3,4,5-trimethoxyphenyl)-N-methylnitrone and selected (E)-2-substituted nitroethenes
Jasiński R., Ziółkowska M. Demchuk O.M., Maziarka A.
Central European Journal of Chemistry, 12, 586-593 (2014) |
|
|
|
|
|
|
 |
|
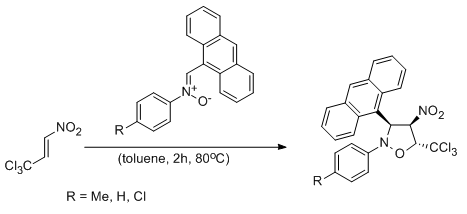
A full regio- and stereoselective synthesis of 4-nitroisoxazolidines via stepwise [3+2] cycloaddition reactions between (Z)-C-(9-anthryl)-N-arylnitrones and (E)-3,3,3-trichloro-1-nitroprop-1-ene: comprehensive experimental and theoretical study
Jasiński R., Zmigrodzka M., Dresler E., Kula K.
Journal of Heterocyclic Chemistry, 54, 3314-3320 (2017) |
|
|
|
|
|
|
 |
|
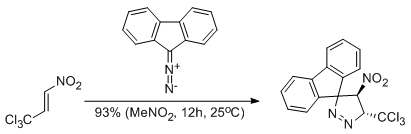
Unexpected course of reaction between (E)-2-aryl-1-cyano-1-nitroethenes and diazafluorene: why is there no 1,3-dipolar cycloaddition?
Jasiński R., Kula K., Kącka A., Mirosław B.
Monatshefte für Chemie - Chemical Monthly, 148, 909-915 (2017) |
|
|
|
|
|
|
 |
|
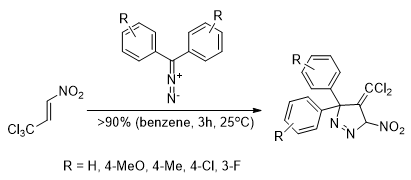
[3+2] Cycloaddition of diaryldiazomethanes with (E)-3,3,3-trichloro-1-nitroprop-1-ene: experimental, theoretical and structural study
Kula K., Dobosz J., Jasiński R., Kącka-Zych A., Łapczuk-Krygier A., Mirosław B., Demchuk O.M.
Journal of Molecular Structure, 1203, 127473 (2020) |
|
|
|
Similar compounds: nitroethene, (E)-1-nitroprop-1-ene, 1-chloro-1-nitroethene, (E)-2-phenyl-1-cyano-1-nitroethene
|
|
|
|
|