|
|
(E) methyl 3-nitroacrylate
|
|
|
|
|
Substance index - selected nitrocompounds tested by our group /
|
|
CAS: 52745-92-3
MW: 131.088
Brutto formula: C4H5NO2
M.p. [oC]: 38 [Ref.]
B.p. [oC]: 72/11mmHg [Ref.]
UV/VIS [nm]: 221 [Ref.]
IR [cm-1]: 1730 1637, 1539 [Ref.]
1H-NMR [ppm]: 7.69 (d, 1H), 7.08 (d, 1H), 3.88 (s, 3H) [Ref.]
|
Preparation [Ref.]:
|
|
Methyl 2-chloro-3-nitro-propionate (167g, 1mole) was added dropwise to a stirred suspension of anhydrous sodium acetate (90g., 1.1moles)
in ethyl ether (200 ml, anhydrous) at 5'. After addition was completed, stirring was stopped and the sodium chloride and sodium acetate were
filtered. The solution was concentrated at reduced pressure until ether and most of the acetic acid had been removed.
The dark red concentrate was distilled in nitrogen at reduced pressure to yield, after removal of acetic acid: (1) volatile product (116.9 g), b.p. 72-76oC/9-10mmHg),
and (2) residue (15.8 g). Redistillation of the volatile product under nitrogen gave methyl 3-nitroacrylate (115.6g).
The residue from the initial distillation was washed with water and extracted with ethyl ether. The ether extract was dried over calcium sulfate and then distilled to give, after removal of ether, methyl 3-nitroacrylate (4.8 g.) and methyl 2-chloro-3-nitropropionate (7.2 g.), b.p. 80-90oC/1-2mmHg). The combined methyl 3-nitro- acrylate totaled 120.4g (91.9% yield).
|
Applications:
|
|
|
|
 |
|
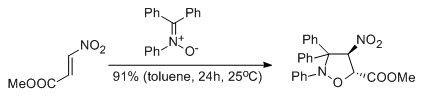
Experimental and theoretical DFT study on synthesis of sterically crowded 2,3,3,(4)5-tetrasubstituted-4-nitroisoxazolidines via 1,3-dipolar cycloaddition reactions between ketonitrones and conjugated nitroalkenes
Jasiński R., Mróz K., Kącka A.
Journal of Heterocyclic Chemistry, 53, 1424 (2016) |
|
|
|
Similar compounds: nitroethene, (E)-1-nitroprop-1-ene, (E)-1-nitropent-1-ene, (E)-1,1,1-trichloro-3-nitroprop-2-ene
|
|
|
|
|